About the author: Victor Miller, EIT, is applications engineer for AdEdge Water Technologies. Ronit Erlitzki, Ph.D., is director of business development for AdEdge Water Technologies. Miller and Erlitzki can be reached at [email protected] or 866.823.3343.
Victor Miller & Ronit Erlitzki
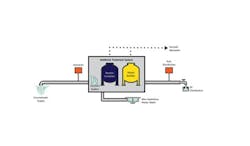
undefinedFull-scale biological filtration system for removal of ammonia from groundwater in Palo, Iowa.
Ammonia has been a silent nuisance contaminant that has gone unnoticed in the water treatment industry for years, and it does not have a mandated maximum contaminant level (MCL). It recently has started to draw more attention due to operator and regulator field observations of high or inconsistent chlorine demand, as well as customer odor or taste complaints.
Groundwater Ammonia Sources
Naturally occurring ammonia, typically considered to be concentrations of up to 0.2 mg/L, may be detected in groundwater due to decomposition of organic materials. However, anthropogenic activity is suspected to be the primary source of the higher concentrations of ammonia detected today (0.5 to 8 mg/L) in aquifers in more than 40 states. These sources include agricultural runoff, industry, disinfection with chloramine and wastewater pollution.
Ammonia becomes a concern due to its interaction with other chemical compounds. As a reduced molecule, ammonia will easily interact with chlorine and oxidize to chloramines, nitrate, nitrite and atmospheric nitrogen. This will result in increased chlorine demand and risk of unsatisfactory residual chlorine in the distribution system.
High chlorine that is added to the water to compensate for the ammonia demand, combined with naturally occurring total organic carbon (TOC), increases the risk of disinfection byproducts (DBP).
Ammonia interferes with a number of conventional water treatment processes that utilize chlorination to oxidize and precipitate various metals. This leads to increased operating costs and the formation of chloramines. These are effective disinfectants, but also are believed to have some toxic effects and can be life-threatening to dialysis patients.
The presence of free ammonia in a distribution system may result in biofouling due to nitrification and increased concentrations of two highly regulated contaminants: nitrate (MCL = 10 mg/L) and nitrite (MCL = 1 mg/L).
Ammonia concentration higher than 0.5 mg/L also is harmful to marine life and can have a serious environmental and economic impact.
Treatment
There are a number of treatment solutions for removal of ammonia from groundwater. They include blending, breakpoint chlorination (BP), ion exchange (IX), reverse osmosis (RO), air stripping, advanced oxidation process (AOP) and biological filtration. While some of these solutions are less effective for ammonia removal, the selection of a specific approach depends on multiple parameters unique to each site, including water quality, application size, space availability, discharge options and operator skill.
Ammonia in groundwater often is accompanied by other contaminants such as iron, manganese and arsenic, and occasionally hardness and TOC. Initial data analysis of raw water quality in different states justifies the relevance of these water quality scenarios.
Biological Filtration Approach
In the municipal drinking water treatment industry, biological treatment has attracted increased attention, as proven by the number of approved biological filtration systems for multiple contaminant removal in a number of states. Biological treatment for drinking water is being added to the 10-States Standards, which is a significant advancement in the drinking water treatment industry.
One such treatment is NoMonia, a biological ammonia treatment process developed and patented by the U.S. Environmental Protection Agency (US 2010/0300964A1) for the treatment of ammonia, based on enhancement of the natural process of nitrification. This dual-stage process consists of a biological contactor followed by a biofilter. The majority of ammonia removal occurs in the first stage of treatment, as the contactor provides optimal growth conditions for naturally occurring nitrifying bacteria. Ammonia not removed in the first stage is removed by the biological filter; the biofilter not only is included as a “polishing” step for ammonia removal, but also acts as a filter for the removal of oxidized metals and other solids that may make it through the first stage.
The first stage consists of a patented aerated contactor, a non-pressurized vessel(s) filled with gravel as a growth medium for naturally occurring nitrifying bacteria, a phosphate injection (phosphate is an essential nutrient for the bacteria), and an aeration system to continuously provide saturated levels of dissolved oxygen (DO). DO provides aerobic oxidation of ammonia that results in complete conversion to nitrate.
As water passes through the vessel, the nitrifying bacteria consume ammonia and convert it first to nitrite and then to nitrate. The contact time within the first stage—empty bed contact time—is a key to all biological filtration applications, due to the nature of mass transfer of oxygen and the kinetics of ammonia-oxidizing bacteria growth. This is a critical parameter for successful operation and typically ranges from 10 to 12 minutes.
The biological filter stage consists of a pressurized vessel(s) filled with filter media for additional biological contact time and filtration of oxidized metals. It has been well documented that a substantial amount of iron, manganese and arsenic can be removed during the first stage of NoMonia treatment, and that the second stage is effective at removing remaining metals to below the MCL. The biofilter’s backwashing schedule depends on the concentration of the metals. A number of demo and full-scale systems have reproducibly shown that a single fully automated dual-stage NoMonia system effectively removes ammonia to non-detected levels, as well as metals to below the MCL without use of any chemicals, excluding small amounts of phosphate.
Cost analysis supports the notion that the system’s operational cost and lifetime system cost over 20 years are the lowest when compared to conventional treatment approaches. NoMonia water recovery is found to be the highest, and the non-hazardous wastewater can be discharged to a sewer line without additional, and costly handling.
Non-Biological Approaches
Despite the advantages of biological filtration, the conventional physicochemical approach for removal of ammonia could be a suitable fit for a specific application; for example, IX or RO could be a solution for a relatively small-scale system with groundwater contaminated with ammonia only, and when there also is access to sewer line for discharge of the high-total-dissolved-solids wastewater.
This information refers to ammonia in groundwater and to a more common situation of groundwater contaminated with ammonia and arsenic, iron and/or manganese.
For BP, chlorine is added in large quantities to oxidize all of the reduced compounds in the water, including ammonia and organics, and metals like iron and manganese. The capital cost for a BP system is relatively low because the primary component is a chlorine-dosing pump. Low capital cost and little to no waste are benefits to utilization of BP for ammonia management. The primary concern with BP is the operating cost due to high chemical consumption, monitoring dosing for residual chlorine, safety concerns and the risk of DBP generation. In this case, the oxidized metals will have to be removed by a downstream filtration process following BP.
Ammonia-selective IX resins are efficient for ammonia removal given that the pH is adjusted to below 7.5 to keep the ammonia at its ionized NH4+ form. These resins are required when competing ions are present in the raw water. The IX resin will periodically require regeneration in order to maintain the ammonia removal capacity, and the concentrated brine waste will have to be approved for discharge by the local regulator, as hauling it away is a costly alternative. IX cannot remove ammonia to concentrations below 0.5 mg/L.
IX resins are sensitive to iron and manganese fouling, so in the case of multiple contaminant scenarios, one solution is a combination of oxidation/filtration (O/F) and IX. The O/F stage likely will be necessary as a pretreatment step to remove metals from the source water. Due to the ammonia in the water, chlorine is not a preferred oxidant in the O/F IX process, and permanganate, which is more costly and difficult to handle, should be used for metals oxidation instead. The filters will require frequent backwash due to potential contaminant buildup, and the IX resin will periodically require regeneration. The O/F IX combined approach generates a high volume of concentrated brine waste (resulting in relatively lower recovery) in addition to creating a higher capital cost due to the multiple treatment steps.
For similar operational considerations, RO will remove ammonia given that pH is adjusted to below 7.4 and readjusted prior to distribution. A typical RO process results in relatively low recovery (80% to 85%), and the concentrated reject stream has to be approved for disposal to a sewer line. Alternative reject stream handling methods, such as evaporation lagoons or hauling away the wastewater, are costly. In addition, the practice of blending the permeate with raw water in order to achieve stability of the effluent (remineralization) prevents the reduction of ammonia by RO to non-detected levels.
RO is not an ideal fit for situations with multiple contaminants. Metal oxidation and precipitation, which can occur even during the pumping of the raw water from the well due to accidental aeration, has the potential to foul the membranes. A pretreatment O/F stage is necessary prior to the RO treatment, which will include removal of the oxidant (chlorine or permanganate) prior to the downstream RO stage, and pH adjustment.
The effluent of the biological treatment NoMonia process—with non-detected ammonia and below-MCL metal concentrations—can be safely directed to IX or RO systems if additional treatment is required.
Ammonia in groundwater needs to be addressed due to the risk of deterioration of water quality. While treatment may become complicated, water professionals can select from a menu of treatment solutions. There is a need for site-specific considerations when addressing ammonia concerns. New technologies are filling in technical gaps in situations where complex water quality exists and regulatory measures are becoming more stringent.