Hot Amine Use: Part 2
Experimental Methods
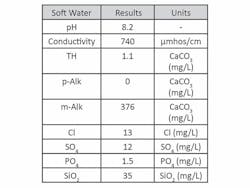
Hot Closed Loop. Coupon testing was performed using a 10-gal, 3,000-watt hot water heater, circulation pump and a CPVC corrosion coupon rack (See Fig. 1, page 11). Each test run lasted 14 days and was maintained at 160°F. The circulation pump provided a flow velocity of 1 ft per second through the coupon rack. While this is lower than the recommended 3 to 5 ft per second velocity, relative data still is recognized to be valuable and quantifiable. Soft water was used to triple rinse the system prior to each run. The rinse water was circulated for 10 minutes at room temperature. Make-up water for each test was prepared separately and pumped into the water heater. Make-up water for all trials remained the same (see Table 1).
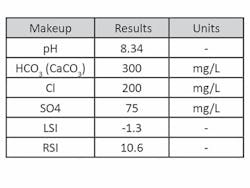
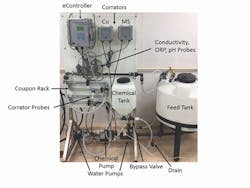
Cold Loop Testing. Filming amine technology also was tested for efficacy in open cooling systems using a small circulating water loop with LPR corrosion probes (See Fig. 2, page 11, and Fig. 3, page 12). Makeup water for each trial was prepared directly in the bulk tank. Analysis of the water analysis is shown in Table 2 (See page 12). The entire system was triple rinsed with deionized water between each run.
Prior to salt additions, 56.78 liters of deionized water was added to the feed tank. Salts were left to mix for 30 minutes in the bypass loop before treatment addition. After treatment addition, the bypass loop was closed to allow flow though the coupon rack and probes. Corrosion data was obtained from Cosasco Corrators located in a coupon rack with a flow rate of 4 ft per second.
Results & Discussion
Hot Closed Loop. A control trial was run to obtain baseline corrosion data for all metals. A pH of 8.2 was chosen as it is within the upper limit proposed by some aluminum heat exchanger manufacturers, and it is in the limits recommended by the Association of Water Technologies for mild steel protection in multi-metal systems. Multiple trials then were performed with a variety of traditional hot closed loop treatments. A nitrite/silicate blend was initially evaluated as a multi-metal corrosion inhibitor at a typical operational pH (i.e., 9.6 pH) for mild steel protection. While mild steel and copper were well protected, nitrite failed to protect aluminum. Results showed aluminum protection is not viable with a nitrite program at its native pH, and may even have had an antagonistic effect on the aluminum due to the high corrosion rate.
Another traditional chemistry, molybdate/silicate, was tested. Test runs were performed at both the naturally derived pH (9.6), as well as a pH adjusted (8.2) run with citric acid. Results, as expected, clearly indicated this combination to be an effective corrosion inhibitor for all metals tested at the lower pH (8.2). While a combination of molybdate and silicate is known to provide aluminum protection, its high performance at pH of 9.6 was unexpected.
In addition to traditional treatments, greener chemistries also were evaluated. Trials 5 and 6 included a commercially available tannin product. Both tannin trials with and without an azole adjunct did not perform well relative to the control under the conditions tested. Interestingly, even at a pH of 9.0, mild steel corrosion was higher than the control with a pH of 8.2. The high aluminum corrosion rates can likely be attributed to higher pH and an increase in iron in the bulk water.
A commercially available filming amine product was tested in trials 7 and 8, which then was compared to a newly developed FFA formulation in trials 9 and 10. Both products initially were tested at the upper recommended dosage of 5,000 ppm as product. At this level, there was no indication of copper or aluminum corrosion in the 14-day trial with either formulation. Mild steel rates were well within the accepted limits, all while operating at lower than desirable pH for mild steel protection.
The results of trial 9 showed a mild steel corrosion rate of 0.03 mils per year (MPY) at a pH of 7.8 within the manufacturer’s recommended limits. Both filming amine blends were tested at the lower end of the usage range (i.e., 1,000 ppm). The commercially available filming amine showed some improvement on mild steel and copper compared to the control, but did not provide aluminum protection at this use level. The results of Trial 10 for the newly developed film forming amine (FFA) at the lower dose were comparable to the results at the 5,000 ppm dose. Only a slight increase in mild steel corrosion is observed, but it was still within the acceptable limits.
As for the aluminum corrosion coupons, in Trial 3, the molybdate/silicate blend served as a benchmark for traditional treatments. Coupons from this trial showed a clean aluminum surface in comparison to the control and tannin trials. The improved performance of the filming inhibitor in Trial 10 is an indication of how viable this technology is.
Cold Loop. Most available filming amine data comes from hot water- or steam-related systems. It previously has shown FFA adsorption increases at higher temperatures. As filming amine technology is being considered for other applications, a study was conducted to determine viability as a corrosion inhibitor in an open cooling circuit. The relatively aggressive nature of the water used in the test (see Table 1, page 10) quickly corroded the linear polarization resistance (LPR) electrodes in the control run, reaching 20 MPY in under four hours.
A dose of 5 ppm FFA significantly reduced initial corrosion rates as compared to the control; however, the trend still indicates an increasing corrosion rate towards the end of the run. The results indicate the FFA provided some level of protection, but the initial dose was not sufficient to adequately film the entire surface. The 10 ppm dose behaved in a similar manner to the 5 ppm dose up to the four-hour mark.
At this point, a steady decrease in corrosion was observed, which indicated there was sufficient FFA present to satisfy the surface demand and form a dense, protective film on the LPR electrodes. It should be noted the results of this test only demonstrated the need to satisfy the demand from all the system surfaces to achieve adequate corrosion protection. The initial dose and time required to achieve protection will vary, as film formation is heavily dependent on surface area, water volume and temperature. Systems with previous fouling, deposition or build-up of corrosion byproducts may exhibit significant sloughing and deposit removal. This also will increase the initial demand of the FFA.
Corrosion data also was monitored for several days after the initial 10 ppm dose to determine the film stability. NaOCl was used to treat the circulating loop and residual Cl2 levels were maintained at 0.2 to 0.5mg/L. As expected, the filming amine did not appear vulnerable to oxidation at the levels of chlorine applied. Corrosion rates were maintained at approximately 1.5 MPY for six to seven days with only the initial dose. Several amine residual tests were taken over the 11-day period after the initial 10 ppm dose.
A rose bengal test method for aliphatic amines was used to measure absorbance at 560 nm with a Hach DR890 spectrophotometer. Three and a half hours after the application of the initial dose, a sample was tested yielding a residual level of 7.96 mg/L. Another sample was tested at 5.5 hours, which indicated 5.03 mg/L of FFA remained—nearly a 40% loss.
A correlation could be seen in the first few days of testing between residual FFA and corrosion rates. Corrosion rates sharply declined as the film began to form in the first two days. From days two to eight, corrosion rates remained relatively steady as residual filming amine slowly decreased to less than 0.2 mg/L. At day seven, a residual of 0.06 mg/L was measured without a noticeable increase in corrosion rate. It was not until the final measurement of 0.03mg/L that a corresponding increase in corrosion was noticeable. Testing indicated that after initial film formation has occurred, residual levels only need to be maintained near the detection limit to maintain corrosion inhibition.
Summary and Conclusions
Comparative testing of corrosion inhibitors was performed in a circulating hot water pilot system of 160°F containing copper, mild steel and aluminum. Efficacy of traditional chemistries, as well as filming chemistries, was determined by metal loss over a 14-day test period. Results clearly show the filming amines were the most effective corrosion inhibitors for all metals tested at the pH range of 8.0 to 8.5 recommended for protection of aluminum. Results using a nitrite/silicate blend showed the difficulty of protecting mild steel and aluminum simultaneously. To effectively protect mild steel, a high pH of 9.6 was necessary. This consequently resulted in a corrosion rate of 378 MPY for aluminum.
A cold water circuit also was used to evaluate the film formation characteristics of a film-forming inhibitor blend. The data showed corrosion inhibition begins with the initial dose and corrosion rates continue to improve over an eight-day period.
There are difficulties encountered when treating multi-metal systems, especially when they contain aluminum components. The results discovered with this testing show FFA offer an effective corrosion inhibitor alternative to traditional chemistries, with possibilities for use in a variety of water systems, including hot, cold, open and closed.
About the author:
Nathan Hardy is associate research scientist at US Water Services Inc. Hardy can be reached at [email protected]. Jim Lukanich is director of applied technology at US Water Services Inc. Lukanich can be reached at [email protected].