SDWA Regulations: Perchloroethylene
Saleha Kuzniewski, Ph.D. is an environmental scientist specializing in remediation and biotechnology research. Kuzniewski can be reached at [email protected].
undefinedThe Safe Drinking Water Act (SDWA) was signed into law December 17, 1974, by President Gerald R. Ford. Legally, it is known as 42 U.S.C. §§ 300f to 300j-26, titled “Public Health Service Act.” When a law is passed and signed by the President, it is recorded in a set of books known as the United States Code (U.S.C.). U.S.C. laws cannot be enforced until new regulations for those laws are issued. These regulations are known as Code of Federal Regulations (CFRs). Congress authorized the U.S. EPA to set regulations for the SDWA and these regulations are known as 40 CFR 141, which includes the National Primary Drinking Water Regulations (NPDWRs), the Surface Water Treatment Rules (SWTRs), and also regulations for groundwater (in subpart S, 141.400 to 141.405). Figure 1 presents a brief history of the legal developments for the SDWA.
The SDWA protects public health by limiting the levels of naturally occurring and man-made contaminants in public water systems (PWSs) and its sources, including rivers, lakes, reservoirs, springs and groundwater wells through regulations in 40 CFR 141. Private wells are not regulated by SDWA. The basic structure of the SDWA is illustrated in Table 1. The PWSs include community water systems (e.g. cities’ water systems), and non-community water systems, namely non-transient non-community water systems (e.g. for factories and schools) and transient non-community water systems (e.g. for gas stations).
As in Table 1, while transient non-community water systems make up the majority of PWS (54.8%), they serve only a small fraction of the population (4% as of 2019) while community water systems serve 94.1% of the population, although they are not as dominating as transient non-community water systems. Although they serve a relatively smaller population, PWS — especially transient non-community water systems — face significant challenges in complying with the SDWA due to poor access to capital and technical resources.
How Do Contaminants Land in the List Under 40 CFR 141?
The U.S. EPA assesses the risks posed by contaminants and their likelihood to occur in PWS. Based on these assessments, EPA identifies which contaminants to regulate.
The potential contaminants, first placed on the contaminant candidate list universe (CCL universe), are then screened according to screening criteria, and the selected contaminants are placed in the preliminary CCL. More evaluations follow from experts leading to the final CCL list. There are several CCL lists such as CCL1 created in 1998 consisting of 60 contaminants, CCL2 created in 2005 consisting of 51 contaminants, CCL3 created in 2009 consisting of 116 contaminants, CCL4 created in 2016 consisting of 109 contaminants, and leading to the CCL5 draft as of 2021.
The audience involved in developing the CCLs have evolved over time and in addition to national agencies, public comments were included starting with CCL3. EPA has a Science Advisory Board (SAB), a chartered federal advisory committee providing scientific review, advice and recommendations to the EPA Administrator for EPA actions. The SAB invited public nominations of scientific experts to review the CCL5, currently in its draft stage.
From the final CCL, the law requires that five or more contaminants for which sufficient data are available must be evaluated by the EPA to determine whether or not they should be regulated using a process called regulatory determination that uses three established criteria based on health risk and effects. For the selected contaminants, the EPA sets a maximum contaminant level goal (MCLG) which, according to 42 U.S.C. § 300g in 1(b) 4(A) is defined as the level at which no known or expected adverse effects on a person’s health occur and which allows for an adequate margin of safety. After setting the MCLG for the contaminant, the EPA then sets enforceable standard called the maximum contaminant level (MCL). The MCL value, while also remaining close as feasible to the MCGL value, also takes practical consideration into account.
The National Primary Drinking Water Regulations (NPDWR), applies to public water systems and consists of legally enforceable primary standards for organic and inorganic chemicals in addition to for microorganisms, disinfectants and their byproducts, and radioactive chemicals.
These standards, presented in the form of a table, include the MCLG and MCL in addition the source of contamination in drinking water, and the potential health effects due to long term exposure above the MCL. General requirements for treatment techniques for compliance to protect public health are also presented for different chemicals. While the online format is easy to follow, updated and complete information on 40 CFR 141 (i.e. the NPDWR) is available on the U.S. Government Publishing Office.
PCE & Its Degradation Products
The organic chemicals in the NPDWR include the difficult-to-remediate chlorinated volatile organic compounds (VOCs), such as perchloroethylene (PCE) and its degradation products through microbially-mediated reductive dechlorination, namely trichloroethene (TCE), dichloroethane (DCE) and vinyl chloride (VC).
PCE was commonly used in the past in dry cleaning and as a degreasing agent for metal cleaning. When discharged through accidental spill or leak from disposal containers, leaking underground pipes, or unlined discharge structures, it migrates within the soil subsurface via three primary mechanisms that occur concurrently: infiltration, diffusion and capillary action.
PCE is a dense nonaqueous phase liquid (DNAPL) due to its high vapor pressure and soil adsorption coefficient as shown in Table 2. Due to the latter, it adsorbs strongly to the soil and via the process of diffusion and capillary action, it can infiltrate deeper soil layers, including clay and eventually into the groundwater because of its high water solubility. PCE, known as a chlorinated VOC because it has chlorine in its structure and is volatile, meaning it has high vapor pressure (Table 2), can partition into a vapor phase in the soil and PCE vapors can enter buildings via advective/convective transport in a process commonly referred to as vapor intrusion.
The partitioning of PCE to the vapor phase from either the sorbed phase (i.e. from the soil) or the dissolved phase (i.e. from water) requires adequate interactions between these two phases and also depends on several factors, including ambient temperature, permeability of the unsaturated zone, and most importantly, on the concentration of the PCE.
The concentration of PCE detected in soil sampled from PCE-contaminated soil is usually lower than the concentration at the soil site and similar observations have been made for PCE-contaminated water samples, possibly due to the PCE escaping as a vapor at the time of sampling or the aqueous PCE “sinking” via diffusion into the unsaturated zone over time. Detected in the sample is a lower concentration unreflective of the actual concentration in the contaminated site.
PCE Remediation
While the low PCE concentration detected in samples makes it difficult to plan remediation strategies for PCE contaminated sites, nevertheless there are biological and physical remediation strategies that have proven effective to comply with the MCL requirements in the SDWA.
The MCL (Table 2) of PCE and TCE is 0.005 mg/L while it is relatively higher for trans DCE (0.1 mg/L) and cis DCE (0.07 mg/L), and lower for VC (0.002 mg/L). The biological remediation of PCE involves reductive dechlorination — an anaerobic process — in which PCE releases one chlorine ion while accepting two electrons from an electron carrier, forming TCE. TCE forms cis and trans DCE and these form vinyl chloride and finally ethene is produced; all from reductive dechlorination.
While aquifers are generally aerobic and thus incapable of reductive dechlorination, there are physical options for PCE remediation including dilution, sorption and volatilization, resulting in the reduction of PCE concentration. PCE is classified as a likely carcinogen by the EPA while TCE becomes a suspected carcinogen after it is chemically processed in the liver after inhalation.
As mentioned above, the reductive dechlorination of TCE produces VC, a known carcinogen. PCE and its degradation products including TCE and VC all have high vapor pressure (Table 2) and thus inhalation of all these chemicals is a major exposure pathway and a health risk.
Purpose of SDWA Analysis
The objective of this article is to critically analyze the regulatory issues for PCE and its degradation products in public water systems as in the SDWA. These include the EPA risk review for PCE, issues with the best available technology (BAT) for PCE remediation, best available evidence for the MCGLs and MCLs, and the EPA’s rule for phasing out PCE in dry cleaning.
Issues Within the NPDWR
Within the NPDWR, there are issues that conflict with another statute or contends with the use of evidence for regulatory purpose, specifically the best available technology (BAT) which does not satisfy the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA) clean-up standards and the contention about the best available evidence for setting MCGLs. These issues are discussed in this section.
Best Available Technology for PCE Remediation
The EPA regulations that enforce the SDWA lists the MCLGs (specifically in §141.50) and MCLs (specifically in §141.61) for PCE and its reductive dechlorination degradation products while also recommending the best available technology (BAT) for achieving compliance with the MCLs.
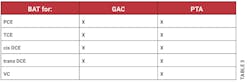
According to the definition in §141.2, BAT means the best available treatment or technology found by the EPA Administrator to be effective under both field and laboratory conditions for helping to comply with the concentrations in the MCLs. This definition also mentions that any BAT must be at least as effective as granular activated carbon (GAC) technology. The BAT technologies for organic contaminants listed in §141.61(b) are granulated activated carbon (GAC), packed tower aeration (PTA) and oxidation. These are not the only BAT technologies, as there are other technologies for other regulated contaminants, such as ozone treatment and enhanced coagulation.
In §141.61, in addition to the MCLs for organic contaminants listed under section (a), section (b) lists the BAT for organic contaminants including for PCE, TCE, cis and trans DCE, and vinyl chloride. The BAT for these VOCs are packed tower aeration (PTA) or oxidation in addition to GAC. As shown in Table 3, PTA is listed as the BAT for PCE and its reductive dechlorination products while GAC is also listed for all except for vinyl chloride and oxidation is not listed for any of these VOCs.
PTA, as the name implies, is an aeration technology and is used in the U.S. in the form of packed towers. Specifically, it is an air stripping technology that moves VOCs from the aqueous solutions into the enclosed air in the tower, and it works on VOCs that have high vapor pressure and low water solubility including PCE, TCE, DCE, and VC. In wells, this aeration technology is used effectively as an air lift or as an electric submersible pump and sparger. For example, at Collegeville, Pennsylvania, the electric pump and sparger effectively removed 82% of TCE and at Glen Cove, New York, air pumps with sparger removed approximately 73% of TCE.
The aeration technology is also used in combination with GAC. However, GAC — which basically is a carbon adsorption technology — has limitations as it has a finite capacity for sorption of chemicals, including VOCs. Also, dissolved organic carbon can compete with TCE for binding site on the GAC. For example, in the presence of 10 mg/L of natural organic matter, TCE adsorption on GAC was reduced by up to 70%.
PTA also has its own legal limitations: since it simply transfers VOCs such as TCE from one medium (i.e. soil or water) to another (i.e. enclosed air in a tower), it does not significantly reduce the volume or the toxicity of the VOC and thus, it does not satisfy the intent of CERCLA cleanup standards (42 U.S.C § 9621). This CERCLA section states that remedial treatments that result in a permanently significant reduction in the volume, toxicity or mobility of the hazardous substance are to be preferred over remedial treatments that do not lead to such reduction.
Bioremediation of PCE and TCE has the potential to fulfill CERCLA’s cleanup standard by reducing the concentrations of PCE and TCE and producing nontoxic products. In the aerobic degradation of TCE, a consortium of microorganisms are involved that produce hydrolysis products from TCE, which are in turn utilized by the microorganisms and in anaerobic remediation, relatively nontoxic chemicals are produced such as ethylene and carbon dioxide. In both of these remediations, the concentration of TCE decreases with the production of the relatively nontoxic chemicals.
However, the BAT as mentioned for PCE and its reductive dechlorination products does not mention bioremediation. It mentions PTA and GAC, although it does say that the EPA Administrator, pursuant to the SDWA identified PTA as the best technology treatment or other means available for achieving compliance of the MCLs.
This indicates that bioremediation could be used as a treatment technique. According to the definition for BAT, as discussed earlier in this article, BAT is set by the EPA Administrator based on the efficiency in lab and field condition, taking cost into consideration, and also any BAT must be at least as effective as GAC. However, this still does not provide any rational explanation for why the BAT for PCE and its reductive dechlorination products do not mention bioremediation as a treatment technique. If it does, it would fulfill the CERCLA cleanup standard.
EPA Risk Review and Best Available Evidence: An Issue for MCGLs
The SDWA requires the U.S. EPA to conduct a review every six years for existing NPDWRs and to determine which regulation(s) if any needs to be revised. In these reviews, EPA requests public comments on the NPDWRs to be reviewed and instructions are also posted for how to send comments. There are several stages of the review, namely review 1, review 2 and so on.
The latest six year review started in 2017 and included PCE, TCE, and cis and trans DCE among eight NPDWRs as candidates for regulatory revision. The six year review 3 process, while very similar to the process for reviews 1 and 2, had more specific clarifications added to it: while the review outcome for PCE, TCE, and VC was “not appropriate for revision at this time” and “no new information”, the NPDWR remains “appropriate after review” for trans DCE, the available new health assessment in this review listed cis DCE as one of the chemicals for potential decrease in MCLG. However as of 2021, the MCLG for cis DCE has remained the same, 0.07 mg/L, the same value as before the six year review that started in 2017 and whether it would change by the end of the six year review (i.e. in the year 2023) is yet to be seen.
The MCLG values, while aspirational, allows for the MCL values to be set. The MCL values takes practical consideration into account while also remaining close to the MCGLs as feasible, as could be seen in Table 2. Therefore, since the 2017 six year review mentioned there is potential for decrease in MCGL for cis DCE, and if the MCGL decreases, then the MCL might also decrease. Inhalation of trans and/or cis DCE causes liver damage and with a relatively high vapor pressure and low soil sorption coefficient compared to PCE (see Table 2 above), meaning more of it is in the gas phase (vapor) and highly mobile in the soil, cis DCE is a health risk and thus, it is imperative that the U.S. EPA review the MCLG value for it based on the 2017 US EPA six year review.
When the SAB is not available, the U.S. EPA uses a nonlinear approach for setting the MCLG and this has been a contentious issue best exemplified by Chlorine Chemistry Council v. EPA (2000). The Chlorine Chemistry Council argued that the U.S. EPA violated its statutory mandate to use the “best available” evidence for implementing the MCLG for chloroform which was zero. The U.S. EPA came up with this zero MCLG value for chloroform in March 1998 using a nonlinear extrapolation approach and assumed there is no safe threshold. The U.S. EPA asserted that using this approach is well-founded, however after considering information from the available SAB, the U.S. EPA stated that it no longer believed it should continue with the zero MCLG value. The MCLG for chloroform, as in the NPDWR (in 40 C.F.R. 141 § 141.53) as of 2021, is 0.07 mg/L.
EPA’s Rule for Phasing Out PCE: A Challenge for Dry Cleaning Operations
PCE, due to its cleaning ability, was a popular solvent in 1962 and was used in 90% of dry cleaning operations in the United States. Among the four grades of PCE manufactured for different uses is a dry cleaning grade manufactured by Dow Chemical (DowPerTM), Vulcan Chemical (PerSecTM), and PPG Industries Inc (PerkloneTM). Since PCE degrades in the presence of light, heat, and oxygen to form trichloroacetyl chloride and tetrachloroethylene oxide, and hydrochloric acid in the presence of water, stabilizers that function as an antioxidant and neutralizer are added to the dry cleaning grade-PCE. While the demand for PCE is increasing in East Asia and is anticipated to grow 2% over the next nine years, however, this demand and the production volume is declining in the United States. In 1991, 83% of dry cleaning machines in the United States used PCE as the main solvent and this value decreased to 60% in 2017.
In addition to the popularity of wash-and-wear fabrics that doesn’t require dry cleaning, the decline in the use of PCE in dry cleaning is mainly attributed to the US EPA’s requirement in 2006, as in 40 C.F.R. Part 63 to reduce the air emission of PCE by phasing out the use of PCE in dry cleaners located in residential buildings by December 2020. The requirement also prohibited new cleaning machines in these residential areas from using PCE and stated that they “must” use alternative cleaning methods. Since residents live in close proximity to the dry cleaners in their residential buildings, they have a higher chance of being exposed to PCE vapors and thus, an estimated higher cancer risk than non-residents. In 1993, there were about 1,300 of such dry cleaners in residential buildings in the United States and this number is higher in 2021 due to the popularity of businesses-residential combo buildings.
These US EPA requirements, among the several other requirements for dry cleaners of different capacities in the United States, were implemented because the Clean Air Act (CAA) requires the US EPA to regulate air pollutants from large industrial facilities and PCE is, according to the US EPA, a toxic air pollutant and a suspected carcinogen. These regulations aim to reduce the emission of PCE into the air: the 1993’s EPA regulations prevented approximately 15,000 tons of PCE from being emitted into the air and the 2006 regulations, including the regulations mentioned above, would prevent another 400 tons of PCE from being emitted into the air. Minnesota became the first state in 2021, following its city Minneapolis in 2018, to legally ban PCE from dry cleaning by passing the bill, HF-91. In this bill, the use of PCE as a dry cleaning solvent would be prohibited starting on the first day of 2026 and dry cleaners would be reimbursed for efforts to transition into using other dry cleaning solvents.
Dry cleaning solvents, other than PCE, include high-flashpoint hydrocarbons and liquid carbon dioxide which have legal issues or disadvantages for uses. The high-flashpoint hydrocarbons used in dry cleaning are specifically petroleum-based solvents composed of aliphatic hydrocarbons and in addition to being highly volatile, they are also highly flammable. Exxon Mobile DF 2000 and Chevron Phillips Eco Sov are commercial examples. The legal issues with high-flashpoint hydrocarbons is that they considered as VOCs by state and federal agencies due to their volatility and they could also be hazardous if they contain benzene. The other type of solvent used in dry cleaning, liquid carbon dioxide, is not used alone and is mixed with costly, specialized detergents under a high pressure of usually 700 psi. The costly detergents is a disadvantage for using liquid carbon dioxide in dry cleaning. Nonetheless, there is a chemical-free technology called professional wet cleaning which uses water as a solvent and generates no hazardous wastes. However, the disadvantage of this is its high cost for its dry cleaning equipment and the need for training operators for cleaning the system. These legal issues and disadvantages for alternatives to PCE are a huge challenge for dry cleaning businesses. Will dry cleaning businesses be able to adapt to the new regulations phasing out and banning PCE? Can other non-PCE solvents and technologies take the place of PCE, which had superior cleaning ability, and still sustain these businesses? Nevertheless, it is imperative to reduce the air emission of PCE by phasing out and banning the use of PCE in dry cleaners since not only is PCE discharged as a liquid effluent and could affect the water quality as regulated by the SDWA, it can also vaporize and this vapor is as harmful to health and thus, the US EPA took this action to protect people’s health and the environment.
Conclusions
The United States has one of the world’s cleanest and most-effectively regulated public water systems, largely due to the SDWA enforced by the US EPA. However as discussed in this article, there are complex issues regarding the regulations of PCE in the SDWA. These include the best available technology that conflict with other statutes or the contentious use of best available evidence for setting standard goals.
Additionally, the EPA’s rule for phasing out PCE affect drycleaners and they need support to transition to the use of PCE-free solvents, some of which could be relatively more expensive that the PCE-based solvents they used and professional wet cleaning. Minnesota stated in its PCE-ban bill that dry-cleaners would be reimbursed for efforts to transition into using other dry cleaning solvent and this support should be used as an example by professional associations to help dry cleaners with their transition.