Semiconductor: Harnessing Diamond Electrodes
Advanced oxidation processes (AOPs) can mineralize recalcitrant organic and inorganic contaminants in toxic and biocidal wastewater streams that are difficult to treat by other means via the creation of hydroxyl radicals. Most practical AOPs use a strong oxidizing reagent such as hydrogen peroxide or hypochlorite. Electrochemical-based AOPs are attractive as they do not require purchase, storage and handling of reactive reagents, and are effective even on relatively small-scale effluent streams. However, electrochemical-based AOPs have been largely unavailable and thus far ineffective as a commercially feasible electrode that can withstand the harsh environment of commercial electrochemical oxidation. Electrochemical oxidation electrodes based on boron-doped diamond (BDD) have been researched for more than 20 years; however, it is only recently that practical BDD materials have emerged, with the oxidation capacity, corrosion resistance and lifetime required for cost effective industrial scale electrochemical AOPs.
Diamond is a semiconductor that can be heavily doped with boron, giving it metal-like electrical conductivity, while retaining its renowned mechanical properties. Organic oxidations require the application of extremely high electrode potentials, at which most electrically conductive material themselves oxidize, including titanium-based dimensionally stable anodes (DSA) and lead dioxide versions. BDD’s inert surface suppresses the electrolysis of water, enabling the required electrode potentials and does not itself oxidize and corrode.
Electrochemical AOPs use DC power to generate hydroxyl radicals directly from the hydroxyl ions in solution. Highly reactive and very short lived, they mineralize dissolved organic pollutants in water.
Electrochemical Processing Grade BDD Electrodes
BDD is not a typical electrode material, and research by material scientists, chemists, physicists and engineers has been required to harness its notable properties. BDD is produced using a form of chemical vapor deposition (CVD), where wafers of polycrystalline diamond are grown outside the normal high temperature and pressure region of the phase diagram for diamond. The extreme overpotential at both the anode and cathode surfaces required the development of a grade of bulk freestanding (i.e., not attached to a substrate) BDD, that is robust enough to survive these extreme electrochemical conditions. Capable of operating at more than 20,000 amperes per sq meter, Element Six’s Electrochemical Processing grade BDD has a proven working lifetime of greater than eight years in operation. To fully exploit these robust BDD materials, an electrochemical cell of bipolar stacked BDD electrodes has been developed. These new electrodes have been incorporated into a non-conductive containment housing, resulting in the full-scale Diamox electrochemical AOP reactor and the laboratory test scale reactor, Diapod.
Spent Caustic Treatment
Electrochemical BDD AOPs are suited to high conductivity and heavily contaminated waste effluent streams. An example of this type of wastewater is spent caustic streams from refineries that have a high chemical oxygen demand (COD) and contain chemicals that are hazardous, inhibitory or biorefactory. The potential chemicals in the spent caustic wastewater include reduced sulfur compounds such as sulfides and mercaptans, as well as organic species such as the sodium salts of naphthenic and cresylic acids. Due to the types of chemicals contained in the spent caustic, this wastewater can be environmentally hazardous and difficult to treat with conventional biological methods.
Electrochemical BDD AOP was identified by Siemens Energy Inc. in Rothschild, Wis., providers of spent caustic treatment solutions as a treatment process that could significantly reduce the COD while producing a biodegradable effluent. Siemens Water Solutions addresses water and wastewater needs of the oil and gas industry with products and services that include physical and chemical separation, biological treatment, and complex hydrothermal technologies for offshore, onshore, upstream and downstream activities. Siemens works with most major oil companies worldwide. Siemens offers a wet air oxidation (WAO) process for treatment of spent caustics. This process may not be cost effective for small refineries. AOP process will address the needs of these refineries.
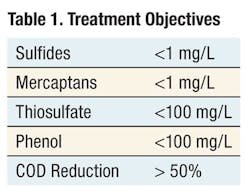
Siemens performed a treatability study for the treatment of various spent caustic from the petroleum refining industry using a 3-kW Diapod electrochemical oxidation reactor. The treatment objectives for the treatability study are presented in Table 1.
The initial treatability study was performed using a sulfidic spent caustic from a refinery product sweetening process. The characteristics of the treatment of spent caustic effluents are presented in Figure 1. The majority of COD for the sulfidic spent caustic was due to reduced sulfur compounds in the wastewater. Following treatment, the minimized sulfur compounds were reduced to non-detectable concentrations, and the COD of the spent caustic was reduced by more than 95%.
A treatability study also was performed using two different mixed spent caustics (sulfidic, cresylic and naphthenic) from a refinery process. The characterization of the untreated spent caustic and oxidation effluents are presented in Figure 2. The majority of COD for the mixed spent caustic was due to organic compounds in the spent caustic. The spent caustic mix also contained lesser concentrations of reduced sulfur compounds, including sulfides and mercaptans. Following treatment, the complex organic compounds, such as phenolics and naphthenic acids, were broken down, which resulted in a significant reduction of COD. In addition, the reduced sulfur compounds were reduced to non-detectable concentrations. The overall COD of the spent caustic was reduced by more than 90%, as shown in Figure 2.
Conclusions
BDD electrode-driven AOPs are a promising electrochemical treatment process for small- to medium-volume recalcitrant dissolved organic waste streams. The electrochemical processing grade of BDD enables industrial scale treatment processes to be developed. A treatability study for spent caustic has found the process to be efficient for treating the effluent stream and mineralizing the recalcitrant species contained within it. Like all AOPs, it is a relatively high cost process that is best used in combination with other treatment processes such as biological treatment to tackle the most difficult effluent streams. With the introduction of this technology, the prospects for BDD electrode-driven electrochemical AOP systems are stronger.
Download: Here