Ever Increasing Algae Blooms & Dead Zones Threaten U.S. Waters & Aquatic Life
About the author:
Tyler Marshall is a principal environmental engineer in the Iowa City office of Stanley Consultants, performing civil and environmental engineering projects since 1998. Marshall can be reached at [email protected].
|
Read the other articles in this series
|
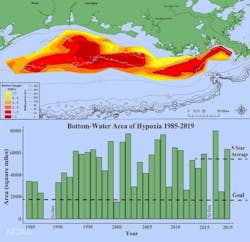
In August 2019, the Gulf of Mexico dead zone at the delta of the Mississippi River was measured at 6,952 square miles, the eighth largest in the 33 years of the now-annual summer occurrence. Fish, crabs, shrimp and other aquatic life mobile enough swam to cleaner waters, while less fortunate ones at the ocean bottom died. There’s nothing for fishing boats to catch. Worse, shrimp in the area are smaller because of the pollution. It’s not just the fishing industry that suffers, it creates numerous side effects on the regional ecosystem.
What is a Dead Zone?
The dead zone in the Gulf of Mexico represents one of the biggest environmental crises of the modern age. A dead zone is an area within a body of water where in connection to algae blooms, dissolved oxygen levels drop to a level where the water can no longer sustain most aquatic life, resulting in large-scale fish kills. In the case of the Gulf of Mexico, the dead zone originates at the mouth of the Mississippi River and has been growing over time. The 2017 measurement put it at more than 7,800 square miles – larger than the state of New Jersey.
Scientists call it eutrophication, when a body of water gets too many nutrients. The algae, or cyanobacteria, grows out of control and uses most of the oxygen needed by aquatic life. The dead zone isn’t limited to the Gulf of Mexico. Many more thousands of square miles of water upstream and in lakes and tributaries are also dead or polluted. The problem is not going away, and it’s getting worse.
Additional harmful algae blooms (HAB) have been occurring in inland rivers, streams, and lakes, causing fish kills, fouling drinking water and closing recreational waters and beaches for extended periods. An algae bloom in Lake Erie in 2014 resulted in elevated levels of microcystin, which is a toxin produced by certain types of blue-green algae. It caused 400,000 residents of the area around Toledo, Ohio unable to drink tap water.
Microcystin can produce hives or blisters from direct contact with the skin. Swallowing it can cause headaches, fever, nausea, vomiting and diarrhea. Large-scale ingestion can damage the liver. The toxins are potentially deadly to pets as well. There have been many cases documented recently where dogs have died within a matter of hours after exposure to algal toxins.
What Causes Algae Blooms?
The potential for extreme toxicity from microsystin causes public health authorities to close recreational waterways and beaches for extended periods of time as a precaution. The August 2019 EPA HAB Newsletter indicates there are more than 253 harmful algae blooms in lakes and beaches, resulting in recreational closures or public health advisories.
Algal blooms result from a combination of environmental factors including available nutrients, temperature, sunlight, ecosystem disturbance, hydrology and the water chemistry. Excess nutrient loading is the most prevalent factor contributing to algae growth. Nitrogen and phosphorus–too much of a good thing is not always a good thing.
In the case of the Mississippi River watershed, the dead zones and algae blooms have been directly traced to abundance of nutrients such as nitrogen and phosphorus that find their way from farms and lawns into the rivers and streams that eventually drain to the Gulf of Mexico.
Nitrogen & Phosphorus in Midwest Agriculture
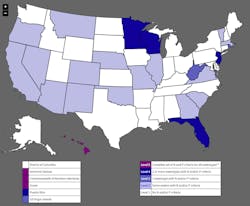
The problem of nutrients is prevalent throughout the nation, but particularly in farmland Midwest. The Minnesota Pollution Control Agency recently labeled 56% of its 3,416 bodies of water as impaired. Phosphorus and nitrate were the biggest culprits. The most striking addition to the list was the St. Croix River from Taylors Falls south to Stillwater, contaminated by phosphorus.
Most of the nutrient loading is created by so-called non-point sources such as soil erosion, agriculture drainage and stream bank erosion, which contribute 92% of total nitrogen and 80% of the total phosphorous runoff. Point sources such as municipal wastewater treatment plant discharges, industrial discharges, and stormwater runoff contribute approximately 8% of nitrogen and 20% of phosphorus entering our watersheds. Once they are in the water, there’s little you can do about it.
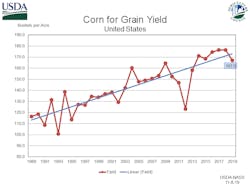
The majority of the non-point nutrient discharges are not a result of ignorance or carelessness but are instead a reflection of the ever-increasing demands being placed on farmland to produce higher yields.
Higher yields come at the cost of increased acres planted, increased use of fertilizers, and increased loss of topsoil. Modern farming practices require a deep understanding of soil chemistry, climatology and the growth requirements of crops to produce optimum yields. However, even the most precise application of the exact amount of fertilizer is subject to the whims of the weather. A cold wet spring can result in nitrogen fertilizer being washed down through the soil column faster than the crop roots can grow and take it up for plant growth until it finds its way into a drain tile and then into the watershed.
The Push for Numeric Water Quality Criteria
The U.S. EPA and individual states are aware of the increasing impacts of nutrient loading on lakes and streams. Many individual states have chosen to take the approach of regulating nitrogen and phosphorus in the same manner as any other water quality pollutant. The pressure to adopt numeric limits is increasing just as the impact of nutrients is increasing.
While numeric criteria are useful in setting clear and well-defined targets for nutrient loading, some critics of this approach argue that it unfairly puts the burden of the nutrient problem on the regulated community—in other words, NPDES point source permit holders such as cities, towns and industries.
On the other hand, the imposition of specific nutrient criteria does allow the state regulators to use additional tools such as classifying a stream as impaired for nutrients, or no longer capable of meeting its designated uses such as potable water source or aquatic habitat. This would further allow the agency to develop total maximum daily load ratings for a given watershed, which is essentially a plan for restoring impaired waters that identifies the maximum amount of a pollutant that a body of water can receive while still meeting water quality standards. This plan opens a larger toolbox of compliance options at the state level.
The Cost of Water Quality & Point Source Reduction
Unfortunately, numeric water quality standards come at a cost. In order to meet the numeric criteria at the point of discharge, individual wastewater treatment facilities would need to construct costly chemical- or biological-based treatment systems. According to the economic impact analysis conducted the state of Wisconsin performed when the state adopted numeric nutrient phosphorus limits in 2010, the cost of compliance was estimated to be between $1.1 and $2.5 billion dollars, adjusted for inflation. Similar statewide cost analysis performed by Iowa estimated initial costs for combined nitrogen and phosphorus point source reductions of between $1.2 to $4 billion. Because water quality standards apply to all dischargers equally, the impact of these costs fall equally on small and large dischargers, affluent cities and impoverished rural communities.
According to EPA studies, it costs in the range of $1 to $10 per gallon of daily design capacity to construct improvements to remove nitrogen and $5 to $10 per gallon of daily design capacity to remove phosphorus. This would mean that for a town of 3,000 people discharging a 0.5 million gallons per day from their wastewater treatment plant, they might expect to have to outlay $5 to $10 million just to comply with nutrient water quality standards. Larger cities may be looking at upgrade costs in the tens or hundreds of millions of dollars. The costs for these upgrades would be on top of the costs of continuing to operate aging infrastructure and remain in compliance with other water quality rules, even as many communities struggle financially.
It can be far more economical to remove nitrogen and phosphorus at its source—non-point field runoff and stream and topsoil erosion. The costs for addressing nutrients through non-point source reductions are estimated to cost between 10 and 25% per pound of nutrient removed. Unfortunately, the current NPDES framework does not allow for point source dischargers to take advantage of these types of non-point source reductions, except for in a few selected locations with approved trading frameworks.
With the nutrient issue threatening to explode, regulators such as the EPA will be forced to adopt stricter nationwide standards that could devastate the budgets of local and state governments and industry. The next article in this series will look at potential solutions that, while still expensive, may cost less and be more desirable to adopt.