Addressing the Impacts of PFAS in Biosolids
In recent years, per- and polyfluoroalkyl substances (PFAS) have become a topic of public concern, particularly when discovered in drinking water supplies. PFAS are a family of more than 3,000 man-made chemicals that have been manufactured and used since the 1940s. This large class of fluorosurfactants have unique chemical and physical properties, which make them extremely persistent, as well as mobile, in the environment.
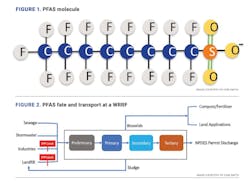
The carbon-fluorine (C-F) bond in PFAS is the strongest bond in chemistry which does not break down naturally in the environment. Long-chain PFAS, specifically perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), have been phased out since 2002. Many replacement compounds are poly-fluorinated and could degrade into precursors of long-chain legacy compounds. The prevalence of PFAS in the environment has raised concerns about the possibility of their adverse health impacts. An illustrative example of a PFAS molecule is shown in Figure 1.
PFAS in Biosolids
PFAS compounds are not directly generated at water resource recovery facilities (WRRFs), but rather enter WRRFs from other sources. Examples include through wastewater generated at industrial facilities that produce or process PFAS, through leachate from landfills that contain PFAS-laden wastes, through municipal wastewater with background levels of PFAS and through contaminated storm water, among others. According to the Interstate Technology Regulatory Council (ITRC), typical treatment methods at WRRFs do not remove or destroy PFAS, and a portion of those compounds may partition to sludge. Figure 2 describes the PFAS fate and transport at a typical WRRF.
Common sludge treatment processes, such as lime treatment, digestion, thermal drying, and composting do not reduce PFAS in sludge. Therefore, PFAS are present in both plant effluent and in biosolids at U.S. WRRFs. The most common PFAS compounds in sludge are PFOS (<10 to 1,100 ng/g dry weight) and PFOA (1 to 240 ng/g dry weight). As expected, the concentration of PFAS in biosolids is higher at WRRFs that serve industrial customers.
In 2004, a North East Biosolids and Residuals Association (NEBRA) survey showed that approximately 55% of wastewater solids are recycled to soils as biosolids, about 30% are landfilled, and about 15% are incinerated. Each of these management options may lead to environmental releases of PFAS. For example, PFAS may transport from landfilled solids to groundwater (unlined landfills) or leachate (lined landfills). Low temperature incineration may cause PFAS to be transferred from the solid matrix to the air. PFAS may also be released from land applied biosolids to the soil matrix, groundwater or surface runoff.
However, the fate and transport of PFAS from biosolids is dependent on several factors. The type and concentration of PFAS must be well understood since the compounds are known to have varying physical and chemical properties. Site characteristics, such as soil properties, weather patterns, plant uptake, and biosolids application methods, impact environmental transport of PFAS. Agencies such as the U.S. EPA Office of Research and Development, the Water Research Foundation (WRF), and several others are investing in research to improve knowledge of PFAS transport and provide guidance to regulators.
PFAS Regulations & Impacts on Biosolids
In 2016, the EPA published Drinking Water Health Advisories for PFOA and PFOS at a combined level of 70 parts per trillion (ppt). In February 2019, EPA issued its PFAS Action Plan that included a goal to move forward with a regulatory determination for PFOA and PFOS limits in drinking water. Many states adopted their own regulations, typically at levels below the 70 ppt health advisory level. Implementation of drinking water limits have impacted biosolids beneficial use programs at WRRFs.
At the federal level, regulations have not been promulgated for PFAS in biosolids. The state-specific regulations and guidelines involve concentration limits of different PFAS compounds in drinking water, groundwater, and, in a few cases, surface water. Only Maine has imposed a limit on three PFAS compounds in biosolids.
As of July 2021, there is no EPA approved method for sampling PFAS in biosolids. Meanwhile, drinking water limits have been imposed on biosolids due to the fear that PFAS may migrate to groundwater. Despite the lack of clear guidance, active investigation of biosolids treatment technologies for PFAS removal is underway.
Treatment Alternatives for PFAS in Biosolids
Several treatment and mitigation options can be considered when addressing PFAS in biosolids. While source reduction is the most cost effective and efficient solution, existing and emerging technologies are available. It is anticipated that future regulatory action will streamline research and development of technologies to reduce PFAS from biosolids. Treatment alternatives include the following.
Source Reduction. The strength of the C-F bond increases the ability to bioaccumulate and not readily degrade. Therefore, the easiest and most cost-effective method to reduce PFAS in biosolids is to mitigate discharges to the WRRF. This could be achieved via 1) reduction of point source discharges from industry; and 2) reduction in background concentrations via drinking water treatment. Both can be achieved through regulatory action, such as setting a PFAS limit via Industrial Pretreatment Programs (IPP), or setting drinking water limits.
Incineration. As of July 2021, thermal destruction is the preferred treatment technology to remove PFAS from biosolids, since high temperatures are needed to break the C-F bond. Incineration is the combustion of residual solids to ash and combustion gas and is commonly used for the destruction of persistent organics from hazardous waste. The EPA is actively investigating the time temperature regimes needed to remove PFAS via biosolids incineration. Packaged dryer-incinerators are also available for on-site biosolids destruction and waste heat recovery at WRRFs. A combined dryer-incinerator could be an energy efficient method to destroy PFAS while generating an end product (ash) that has lower mass and volume. However, incinerator systems are expensive and must meet stringent air permitting requirements. A photograph of a biosolids incinerator stack is shown in Figure 3.
Several emerging sludge treatment technologies exist that could meet the goal of reducing biosolids disposal costs and PFAS mitigation. Emerging technologies include pyrolysis, gasification, and supercritical water oxidation and are described in more detail below.
Pyrolysis/Gasification. Pyrolysis is the thermal decomposition of materials at high temperatures (greater than 450 to 500°C) in the absence of oxygen. The process involves treating dried sewage sludge in a closed pressurized high-temperature reactor vessel. The final products include pyrolysis gas (H2+CO+CO2+others), bio-oil and biochar. The benefit of pyrolysis is that it further reduces the mass of feedstock and products can be used for energy production (gas, oil) or agricultural land application (biochar). The disadvantages of the technology are that it is relatively unproven with sludge in the U.S., that it is has a higher cost than other thermal technologies, and that the operating temperatures (450 to 600°C) are lower than the combustion temperatures of most PFAS compounds (greater than 1,000°C). There are ongoing studies however, that are evaluating pyrolysis for its PFAS destruction potential.
Gasification is the thermal decomposition of materials at high temperatures in the presence of low oxygen levels. Process temperatures are typically higher than pyrolysis, and the products are limited to syngas and biochar/ash. Pyrolysis and gasification have been claimed as methods to reduce PFAS from biosolids. However, further research on fluorine mass balance is needed to understand how PFAS degradation products partition to products.
Supercritical Water Oxidation. Supercritical water oxidation (SCWO) refers to the process that leverages supercritical water for complete oxidation of organic compounds in sludge. In the presence of oxygen, organics can be converted to clean water, inert gases, mineral salts and reusable heat.
There are several benefits to SCWO. First, nearly 99% of the incoming solids are reduced in seconds. Second, the process can be fed with sludge at 10 to 15% solids. Third, the carbonaceous end product is carbon dioxide, the nitrogenous end product is N2 (temperature is too low for NOx), and the sulfurous end product is CaSO4 (temperature is too low for SOx), so air permitting concerns are minimal. Finally, the system has been shown to break the C-F bond in PFAS.
The drawback of the system is that there are no known commercial installations at WRRFs. Additionally, there are likely high energy demands to start the system (though the reaction is self-sustaining after startup).
Biosolids Cost Impacts of PFAS
A collaborative team, including CDM Smith, NEBBRA, WEF and NACWA, completed the Cost Analysis of the Impacts on Municipal Utilities and Biosolids Management to Address PFAS Contamination. The research team surveyed WRRFs, biosolids management companies, and end use facilities (incineration, composting, landfills, farms) to estimate the cost and operational impacts from pending or active PFAS regulations.
When the study was published in 2020, the results showed that in areas where PFAS regulations have been implemented, biosolids management costs have increased by an average of 37%. As more data from those same surveyed facilities has been collected in 2021, the management costs at WRRFs with PFAS regulations have increased to an average of 72%. Some of the states which have seen the greatest impact include Arizona, Michigan, Maine and New Hampshire.
The rapid cost increases have been attributed to reduced land application availability due to PFAS and to the use alternative high-cost disposal sites such as landfills.
Meanwhile, cost impacts have been minimal at WRRFs that manage their own biosolids, do not rely on beneficial reuse, and/or at WRRFs located in areas without PFAS regulation. These results clearly show that local, state, or federal PFAS regulations have a significant impact on the costs of biosolids management.
Summary
Many WWRFs in the U.S. are proactively evaluating solutions to mitigate PFAS. Source reduction is the most cost effective and efficient solution, and several WRRFs in New England are working are considering PFAS limits on industry. Additionally, it is anticipated that drinking water limits will reduce the background concentration of PFAS that enters WRRFs.
In areas with already limited land application or landfill capacity, such as New England, WRRFs are currently evaluating incineration as a viable disposal option. However, in these areas, incineration capacity is lacking, which drives up costs. As a result, WRRFs are evaluating methods to reduce the total mass of biosolids that must be managed, such as sludge drying. Implementation of sludge drying offers the potential to produce a low volume, Class A biosolids product that could be cost-effectively transported to states with less strict land application requirements. Several WRRFs in the Midwest are also considering sludge drying to regain control and flexibility in their biosolids management programs.
To date, there are few known installations of emerging technologies such as pyrolysis, gasification and SCWO. It is anticipated that future regulatory action will streamline research and development of technologies to reduce PFAS from biosolids.
About the author:
Johnathon Sheets, PE, PhD, is an environmental engineer for CDM Smith. Sheets can be reached at [email protected]. Maddison Ledoux, EIT is an environmental engineer for CDM Smith. Ledoux can be reached at [email protected].