Observations of a pH Field Guy
About the author:
Fred Kohlmann is the midwest product marketing manager for analytical products for Endress+Hauser. Kohlmann can be reached at [email protected].
As a field service technician traveling to customer sites to do repairs, there are varied complains to address. They range from component level repairs on circuit boards to sensor replacement, cleaning and calibration. Below are some stories from an Endress+Hauser technician about repairs in the field.
The Light Went Out
A customer called for service and training to be performed on a pH system, including sensor cleaning and calibration. Upon arrival at the site, the technician came with the usual toolboxes of spare parts, buffers and cleaning agents. The customer directed the technician to the location of the pH sensor and indicated it seemed to be working correctly, but the light bulb had burnt out. The technician assumed the customer meant the power or alarm light on the transmitter, but in reality the bulb in the sensor had gone out.
The customer proceeded to pull the sensor up from the tank and showed the technician the measuring end of the sensor. He said this was the light bulb that burned out. The technician explained that this was not a light bulb, but rather the measuring electrode, and it does not light up.
The customer argued he had seen it lit up in the past and due to recent erroneous pH readings and the lack of light, it must be defective. Cleaning and recalibration got the device back running.
If there is a lesson to be learned here, it is that one needs some grounding in the basics before jumping to conclusions, whether it is a pH sensor or any other item in an industrial automation system.
If It Ain’t Broke, Still Calibrate
A pH system was a year or two old and working fine, however, the customer wanted it checked because it was the final, critical control for their wastewater outlet.
An Endress+Hauser technician got to the plant and while walking to the site asked the customer how often he cleaned or calibrated the pH sensor; he said “Never.” He did not want to touch it as it had been working so well since it was installed, and he believed in the “If it ain’t broke, don’t fix it” principle.
“Well, that sure is a good track record, maybe we can use you as a success story for the robustness of our products,” the Endress employee said.
The technician and its customer got to the installation point and pulled the sensor out for inspection. To his amazement, the protective cap was still attached. The protective caps are affixed by the factory to the end of each pH sensor to keep the glass bulb wet and protect it from accidental damage during shipping and storage. It looked like the rest of the sensor body, dirty and crusty. The technician asked the customer if he had put the protective on knowing the company was coming out.
“What protective cap?” he replied.
Most manufacturers put a boot or cap on the measurement end of the pH sensor to keep it safe with a wetting or storage solution of KCl or buffer or a mix of the two. In this case it was a pH 7.0 buffer. The sensor read a perfect 7.0 pH for the many months he had it in use. Now that he was thinking about it, that the amounts of reagents he used to treat his wastewater had dramatically decreased since he installed this new pH system was strange.
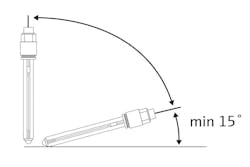
Storage tip: Make sure the sensor is always kept in a storage solution of pH 4, pH 7 or tap water when not in use. Use the protective cap provided by the manufacturer, or place the sensor without the cap in a clear beaker with the storage solution such that the bottom 1 or 2 in. of the sensor are fully submersed. The clear beaker is used so one can see if the solution has evaporated.
Keep the pH Sensor Wetted
Customers often contact manufacturers complaining that their pH sensors drift. Most often it is not the fault of the sensor itself, but is instead due to improper sensor installation, cleaning or calibration.
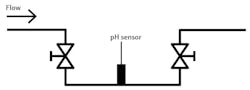
Many pH installations have the sensor mounted into a pipe run, either in a main or bypass line. Depending on the physical layout of the pipe, the sensor location can be tricky. When a customer plans ahead, they put the sensor in a convenient location for maintenance and calibration.
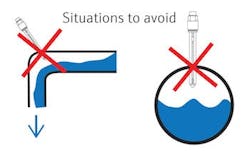
Sometimes that accessible location is in the exact wrong spot for the sensor to remain in contact with the process media being measured at all times. A typical bad location is an installation with the sensor in the top, at an elbow or in a tee in a downward-flowing line of the pipe. Each of these examples allows air to be trapped should the pipe not be full of process fluid.
Some customers swear the pipe is full, and the sensor is just not responsive to the changes in pH values. Many times, technicians have asked for the process to be stopped at the sensor location. Hopefully, the customer had the sensor in a slip stream and used blocking valves to isolate it. When the flow was stopped and the sensor removed, it often is bone dry.
Sometimes technicians were able to unscrew the sensor as the process was running past it, in effect burping the sensor by letting some of the process media seep out, and then tightening down the sensor again. The sensor started reading correctly because the action of burping the sensor when it was partially removed bled air from the line, allowing the sensor to again become wet and read the pH. This will last until a slug of air comes down the pipe again isolating the sensor tip from the wet process.
The pH Sensor Drifts or Does it?
A salesperson and employee visited a customer having problems with a pH system. When they arrived, the customer complained the pH sensors drifted. The two apologized and asked to see the sensor.
The customer had six identical tanks with one pH sensor on each tank. The technician went to the first tank and observed the pH reading. The customer called down to the control room to verify what the pH value should be based on their recipe. The sensor read about 1 pH off.
The customer saw this as validation of the drift because he had calibrated the pH sensor the day before they had arrived. The technician and salesperson asked the customer to show them how the sensor had been cleaned and calibrated.
He pulled the sensor out, rinsed it with water and put it in buffers. In the 7 buffer it read 6. The customer looked at the Endress employee with scornful eyes and put it in the 4 buffer and it read 3. He put the sensor back in the 7 buffer and was about to hit the calibrate button when the Endress technician asked how he would clean the sensor.
He looked at the employee like they had two heads and said he just did. The technician explained that rinsing it did not clean it. The customer looked at the employee strangely and said, “I didn’t know you could clean it any other way.”
The technician said that the process at this plant likely has lots of fats, oils and grease, and cleaning the sensor could be as easy as a drop of liquid dishwashing soap in a cup of warm water.
They immersed the sensor in soapy water, swirled it around and dipped a paper towel in to gently clean the electrode area. They then put it back into the 7-buffer solution and it then read 7 pH. The customer looked at the technician like he was a magician. They rinsed off the sensor and placed it in the 4 buffer and it read 4 pH.
They returned to tank number two and three and had the exact same scenario and exact same results. They were ready to go to tank four and the customer said, “I got it, thank you very much, we learned a lot today.”
It always feels good to leave a customer site after they were able to fix issues and get the process back up and running. In most cases, these problems can be avoided by following manufacturer operating and installation information, and keeping the pH sensors clean and calibrated.