What are Per- and Polyfluoroalkyl Substances (PFAS)?
Per- and polyfluoroalkyl substances (PFAS) are a family of man-made chemicals related to several consumer products, most notably Teflon, non-stick packaging and fire-fighting foam. PFAS are found in water, and have also been found in human blood around the world due to the ubiquitous use of consumer products that have used the chemicals.
The U.S. EPA released a final version of the Fifth Unregulated Contaminant Monitoring Rule (UCMR 5) in December 2021, which established nation-wide monitoring for 29 PFAS chemicals and lithium. The monitoring data will be used to understand how frequent and to what degree these specific PFAS are found in U.S. water supplies to establish further guidance and regulations for treatment.
Definition & Chemical Composition of PFAS
PFAS by chemical definition from the Organization for Economic Co-operation and Development (OECD), are a group of substances with at least one perfluorinated methayl group (-CF3) or a perfluorinated methylene group (-CF2).
They have the general formula R-CnF2n+1 or R-CnF2n-R’ where R and R’ are functional groups or some other groups or atoms besides carbon and fluorine. This accurate definition is discussed in depth by the OECD and it showed that the general definition of PFAS as highly fluorinated aliphatic substances containing one or more carbon atoms is not accurate as it could refer to other substances besides PFAS.
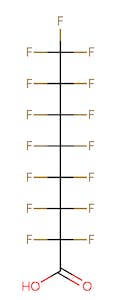
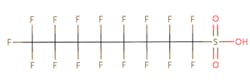
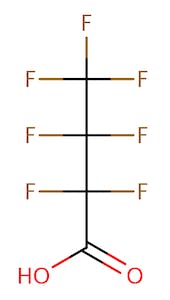
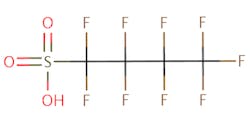
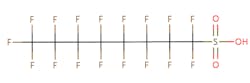
The chemical structures of some of the PFAS, fitting the above definition precisely, is provided in Figure 1 and include perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorobutanoic acid (PFBA), and perfluorobutane sulfonic acid (PFBS).
Figure 1. Chemical composition of Perfluorooctanic Acid & Perfluorooctane Sulfonic Acid
There are thousands of different PFAS that could be classified as short or long chain PFAS. This classification depends on the number of carbon atoms in the PFAS structure. Specifically, long chain PFAS have six or more carbon atoms in their structures. PFBA and PFBS are examples of short chain PFAS while perfluorononanoic acid (PFNA) and the two most commonly studied, PFOA and PFOS, are examples of long chain PFAS. The chemical structures for these are also in Figure 1 above and Figure 2 below.
Are PFAS Dangerous?
According to the US EPA, exposure to PFAS is associated with negative health effects and they can also bioaccumulate in the upper trophic food levels.
The most recent and consistent findings for PFAs health effects in humans include increased cholesterol levels, low birth weight in infants, thyroid hormone disruption, and also increased risk of prostate, kidney, and testicular cancer. Research is still ongoing to better understand how the different concentrations of different PFAS can lead to the health effects especially in children, and this is complicated due to the presence of thousands of different PFAS in the environment. Among the PFAS, PFOA and PFOS are known to bioaccumulate mostly in freshwater and saltwater fish (specifically called the teleosts and these include the sockeye salmon) although they are also found in shrimps and crabs.
The Integrated Risk Information System (IRIS), a database maintained by the U.S. EPA, mentions toxicity values for health effects from chemicals, specifically the reference concentration and reference dose. The only PFAS available in the IRIS database and added recently are PFBA, PFNA, perfluorodecanoic acid (PFDA), perfluorohexanoic acid (PFHxA), and perfluorohexanesulfonic acid (PFxS). Assessment is under development for these.
Currently, there are gaps in existing data on the health effects of PFAS because there are thousands of these chemicals. In October 2021, the US EPA announced that it is developing a national PFAS testing strategy to help it to identify and select PFAS for additional testing. Specifically the US EPA will use this testing strategy by exercising its authority in the Toxic Substances Control Act (TSCA) section 4 to help it to select PFAS for additional testing by requiring PFAS manufacturers to conduct and fund these studies.
The EPA’s recent draft document in November 2021 contained recent scientific data and new analyses and was submitted to the Science Advisory Board. It indicated that toxic health effects from PFOA and PFOS may occur at lower concentrations than previously understood and the finding that PFOA is a likely carcinogen.
PFAS are considered dangerous due to the health effects associated with their exposure, their bioaccumulation in trophic organisms including fishes, and the EPA’s recent finding that PFOA is a likely carcinogen, all as discussed above. Exactly how dangerous they are will be answered by the EPA’s findings in the near future. As more scientific data is available and analyzed by the EPA, more information will be available on the toxicity and carcinogenicity for the different PFAS including for GenX chemicals which are used as replacement chemicals for PFOA.
Where can PFAS be found?
PFAS are widespread in the environment. They are manufactured chemicals historically used in industrial products including in aqueous film forming foam used as a fire suppressant and also found in various consumer products including in stain resistant carpets.
Is PFAS in Bottled Water?
Yes, PFAS is found in bottled water. A published research study authored by John Hopkins University and Stantec in Washington, D.C, found PFAS in bottled water, although it did not identify the brands.
Purified water (water filtered by reverse osmosis) contained less PFAS than spring water. The concentration of PFAS in the bottled water samples ranged from 0.17 parts per trillion (ppt) to 18.87 ppt, with a median of 0.98 ppt. Of the samples, 97% had a concentration below 5 ppt.
This is a lot lower than the value set by the EPA: The US EPA’s Health Advisory for the combined concentration of PFOA and PFOS in drinking water is 70 parts per trillion. These levels are based on the reference dose (RfD) which is an estimate of the amount of a chemical the human population can be exposed to daily without any harmful effects during a lifetime and it is generally expressed in mg per kg of bodyweight per day. The RfD is a reference point used to determine the potential effects of the chemical at other doses. The oral noncancer RfD for both PFOA and PFOS is 2x10-5 mg/kg/day.
RELATED: Read More PFAS Content
Is PFAS in Tap Water? in Wastewater?
Yes, PFAS are found in both tap water and wastewater. The Environmental Working Group (EWG) has an interactive map documenting PFAS contamination in water systems including in drinking water.
Tap water is used as drinking water and in the above link, the different colors can be clicked on/off to show PFAS contamination in different types of water. The blue dots represent PFAS contamination in drinking water and the map shows that most states have PFAS in their drinking water. A similar map but with less data is also available from Aquasana.
Looking at the states, Michigan has a PFAS response website with a map showing PFAS contamination in wastewater treatment plants. As it shows, this contamination is widespread throughout Michigan.
In September 2021, the EPA announced plans for regulating wastewater pollution and this included concentration limits for PFAS discharge in wastewater. While a map similar to Michigan’s is not available for all the states, this announcement by the EPA indicates that PFAS in wastewater is a concern as it can spread into drinking water and thus, it is necessary to set effluent limits for how much PFAS can be in the wastewater.
Is PFAS Organic or Inorganic?
In general, the difference between organic and inorganic chemicals is simple but not always clear. Organic chemicals contain covalent C-H bonds and may also contain oxygen while most inorganic chemicals do not have a C-H bond.
Examples of organic chemicals include sucrose, DNA, and methane, while sodium chloride, phosgene, and carbon dioxide are examples of inorganic chemicals. Hydrogen cyanide is an inorganic chemical because while it has a C-H bond, it also has a C-N bond. As these examples show, the differences are not always clear and adding to the confusion is some definitions that mention that organic chemicals are associated with living things while inorganic chemicals are associated with non-living things. This is inaccurate as proven from the above examples: while methane could be produced from living organisms, so can carbon dioxide which is inorganic.
So what should we look out for when distinguishing between organic and inorganic chemicals? There is no formal definition and different perspectives are used to distinguish between organic and inorganic chemicals including the differences in chemical properties such as water solubility.
However to answer the question, here below follows a perspective on the attributes of organic chemicals that are not found in inorganic chemicals:
- the presence of (1) covalent C-H bonds that form all of the chemical (e.g. methane), or
- (2) covalent C-H bonds that form the majority of the chemical and the structure could also contain oxygen and/or other elements, or
- (3) carbon atoms linked to each other (C-C) to form a chain or a backbone that also contains other elements (e.g. DNA).
The presence of other elements in the third option is important, considering that diamond and graphite are inorganic chemicals. Both diamond and graphite contain only carbon atoms linked to each other in a manner to form uniform structures. They are inorganic because according to (3), they do not have any other elements besides carbon in their structure.
PFAS are organic chemicals as they fit the third option above. As seen in Figure 1, they have carbon atoms linked to each other to form a chain that also contain other elements, prominently fluorine.
Is PFAS a Volatile Organic Chemical?
Although both PFAS and volatile organic chemicals (VOCs) are organic as they fit (3) above, they have different chemical structures.
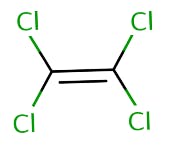
The carbon-fluorine (C-F) covalent bonds are predominant in PFAS while VOCs have carbon-chlorine (C-Cl) covalent bonds. They also have different physical properties, as shown in Table 1 on the differences between PFOA (a PFAS) and tetrachloroethylene (a VOC). PFAS are also not volatile like VOCs due to differing boiling points, vapor pressure, and the organic carbon partition coefficient.
Volatile chemicals like TCE have a lower boiling point, high vapor pressure and high organic carbon partition coefficient than non-volatile chemicals like PFOS and this could be seen in Table 1. Therefore, PFAS are not VOCs.
PFOS
TCE
Structure
Physical description at room temperature (25oC)
Molecular weight (g/mol)
500
Water solubility at 25oC in mg/L
Melting point (oC)
Boiling point (oC)
Vapor pressure at 25oC (mm Hg)
Log Koc (organic carbon partition coefficient)
How Can PFAS be Treated?
There are several treatment technologies for the removal of PFAS on the US EPA’s Drinking Water Treatability Database (TDB). This covers several treatment processes and their effectiveness in bench, pilot, and full scale studies on several types of water including groundwater, surface water, and municipal water.
Additionally, the EPA’s Drinking Water TDB also has a link, when clicked on the treatment process, leads to the option of downloading data to get more specific information on the respective studies. Table 2 summarizes the technologies from the US EPA’s Drinking Water TDB that are proven to be effective in full-scale studies for removing PFAS from several types of water sources.
|
Technology |
Water source tested for full-scale studies |
Effectiveness for removing various PFAS including PFNA |
|
Granulated activated carbon (GAC) |
Groundwater and surface water |
>90% |
|
Ion exchange |
Groundwater |
>40% to >70% |
|
Membrane filtration |
Other water type |
Up to 51% |
|
Reverse osmosis (RO) |
Other water type and municipal water |
>87% |
While Table 2 only mentions the effective technologies in full-scale studies, there are other technologies proven to be effective in pilot or bench scale studies. These include adsorptive media technologies using magnetic nanoparticles and also mixed minerals and graphene-based materials proven effective in bench-scale studies on municipal, lake, and groundwater for greater than 90% removal of various PFAS.
While also effective at removing greater than 90% PFBA, point-of-use granular activated carbon (GAC) devices (i.e. filters installed at a single faucet or fixture and that contains the GAC technology) were mentioned in the US EPA’s Drinking Water TDB to be pilot-tested, even though they are commercially available and some are certified, with their certification mentioning they are effective in removing PFOA and PFOS.
Other bench scale technologies in the US EPA’s Drinking Water TDB proven effective in removing greater than 50% various PFAS including PFBS but only tested at the bench-scale level on municipal and groundwater includes electrochemical, coagulation, and plasma treatment.
As with any technology for removing chemicals from water, the above mentioned technologies are also affected by several chemical factors present in the water. For example, the presence of organic matter in the water can reduce the effectiveness of the GAC technology.
Drinking Water Treatment Technologies for PFAS
Technologies tested on municipal water and proven effective in removing PFAS could be used as drinking water technologies because municipal water is a public water system or water supply network and includes water treatment facilities and pipe network for water distribution. This water distribution system includes drinking water to residential homes. Based on the above discussion and on Table 2, this technology is RO for the removal of various PFAS from drinking water.
Point-of-use GAC is another technology for the removal of PFAS from drinking water and while it has only been tested on the bench scale, according to the US EPA’s Drinking Water TDB, it is used together with RO in commercially popular filter products for residential drinking water treatment.
Several such filters are NSF-certified for removing PFOA and PFOS from the drinking water. However, there are only a handful of such filters and the majority of filters on the market do not or only partially remove PFAS. Filters that lead water into household pipes, such as whole house filtration systems, can potentially make the water quality worse if they remove disinfectants leaving the home pipes susceptible to microbial growth. The drawback of all filters is that they could be costly and need to be replaced periodically.
Wastewater Treatment Technologies for PFAs
GAC and RO are also commonly used together with another technology, nanofiltration (NF), to remove PFAS from factory wastewater and municipal wastewater. Some solution providers for water and wastewater treatments recommend the use of ion exchange as another technology along with GAC, RO, and NF while other providers add to this list including PFAS destructive technology. This technology uses indirect, convective heating to dry the sludge in a dryer cabinet and under a vacuum (to minimize odors). This technology results in a reduced volume of waste along with oxidized contaminants including PFAS and secondly, the heat produced by the biosolids can also be used by the dryer. The oxidized PFAS can then be removed by other technologies.
Biological destruction is another technology which utilizes ozone or a bioactive filter to lower the concentrations of organics in wastewater. High concentrations of organics can interfere with the performance of RO membranes and GAC. By using the biological destruction technology to lower their concentration, RO and GAC are then more effective in removing PFAS from the wastewater.
The list of technologies used together to remove PFAS from wastewater can grow: along with GAC, NF, and RO, some wastewater treatments facilities also use ion exchange. However, its use depends on the presence of inorganic salts such as magnesium, sodium and potassium salts in the water as these can interfere with ion exchange.
In short, a holistic approach is needed to treat PFAS in wastewater: identifying the different chemicals and their concentration in the wastewater, understanding how these chemicals interact with each other, what are the different treatment options and how they can be used as a set, and the optimizations required in the set for efficient removal of PFAS from the water.
Wastewater contains several chemicals that are known to reduce the efficiency of treatment technologies and thus, a set of technologies rather than individual technologies, as discussed above, is required for the removal of PFAS from wastewater.
About the Author
Saleha Kuzniewski
Saleha Kuzniewski, Ph.D. has authored several publications in the fields of scientific research, biotechnology, and environmental regulations. She is the winner of the 2023 Apex award for publication excellence. She is also the founder of Environmental Remediation & Innovations, LLC. Kuzniewski can be reached at [email protected].